1. Not Quite Perfect Superheroes: Essential Life-Giving Substances
At first glance, water seems like the perfect superhero molecule. The molecule's properties are quite special. Fairly resistant to temperature changes, large bodies of water slowly absorb or release heat to the atmosphere and nearby coast. During photosynthesis, water can lose electrons to excited chlorophyll molecules, creating oxygen for respiration and acidic ions that set up a potential difference invested in storing energy. It is a remarkable solvent, dissolving important ions, proteins and sugar molecules
.

But it's not always helpful or innocuous. It can kill or cause brain damage by filling lungs. Its undiscriminating abilities as a solvent allow toxins to tag along on its way to drinking sources and bodily fluids. Other pure substances that fall into water's category include iron, nitrogen, oxygen, sodium chloride and phosphates.
Aside from serving as a raw material for amino acids, relatively inert nitrogen is needed in the atmosphere to attenuate the strong oxidizing abilities of oxygen. Invert proportions of nitrogen and oxygen in the air, however, and the higher concentration of oxygen would lead to an explosive atmosphere. Yet placing oneself in a room with nearly pure nitrogen would lead to suffocation. Sodium chloride, a compound containing two ions essential to animal life, reminds us that the poison is the dose.
If a child ingests about four tablespoons of rock salt, it will lead to acute toxicity. For most plants a much lower concentration is lethal. Phosphates, a component of natural and synthetic fertilizers are needed to assemble ATP and DNA, but in excess they can lead to the eutrophication of lakes.
2. Neither Good nor Bad Substances: Depends On Which Life Form's Point of View
Potato plants, not only produce those familiar, edible tubers but also poisonous berries filled with alkaloids (solanine and chaconine) that protect them from most herbivores and fungi. Even tubers produce poisons when exposed to light. Some varieties investigated for possible cultivation had to be rejected because, even when underground, their tubers produced excessive toxins . From the point of view of the potato, the alkaloids increase its chances of survival--- poisonous for us but essential from the plant's vantage point.
The tough matrix consisting of lignin and cellulose, commonly known as wood, is useless to a starving man, wonderful to a builder, and of course a highly supportive material for the living tree. Aside from the main constituents of wood, there are also aromatic compounds that offer additional protection from insects. But some of these compounds, at least in cases of excessive occupational exposure to hardwood dust (more than 8-hour time-weighted average 5 mg/m3 ), nasal cancer could result.
3. Either Good or Bad Substances: Depends On How Humans Use Them
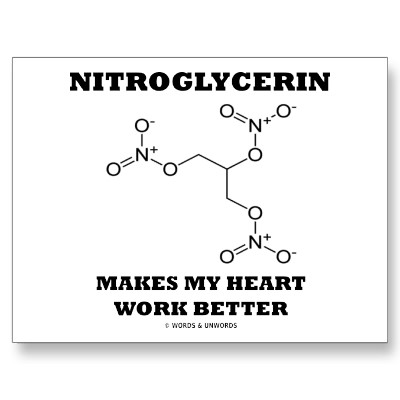
The classic example of nitroglycerin reveals how the impact of some molecules is the result of individual choices. In small quantities it can be used as a vasodilator to treat angina. It can be an ingredient of dynamite, which in turn can be used to either build roads or to wreak havoc. In nitroglycerin's category are many drugs which, regardless of legal status, when used appropriately, lead to favorable outcomes.
 When indigenous South Americans chew on coca leaves, the small amount of cocaine present helps them adapt to higher altitudes. It can also be used as an anesthetic in nose, throat and oral cavity surgery.
When indigenous South Americans chew on coca leaves, the small amount of cocaine present helps them adapt to higher altitudes. It can also be used as an anesthetic in nose, throat and oral cavity surgery. But if cocaine is heavily relied upon for recreational purposes it can become psychologically addictive, and in pregnant women it is passed on to the fetus, leading to developmental complications.
4. Supervillains: Substances That Would Have Been Best Left Unsynthesized
If a chemist reacts elemental mercury with methyl iodide and elemental sodium, he will produce two substances. One, sodium iodide, contains ions that would fall into our essential substance category. But the other compound, dimethyl mercury is one of the strongest known neurotoxins. A concentration of only 5 ppm (0.005 g of poison per kg of body weight) is considered lethal. Although it can be used to calibrate NMR instruments for mercury detection, less toxic salts can be used as
 substitutes, and presently, its use in research is widely discouraged.
substitutes, and presently, its use in research is widely discouraged.In 1995, Karen Wetterhahn, a Darmouth College chemist specializing in toxic substances, accidentally spilled some drops of dimethyl mercury on her latex gloves. Before removing them, she cleaned up the area in the fumehood. She had been taking the recommended precautions. Whoever wrote up the materials safety sheet(MSDS) for dimethyl mercury at the time was unaware that dimethyl mercury can penetrate latex, neoprene and PVC. The MSDS sheet reportedly vaguely, " Wear appropriate chemical-resistant gloves." But once it finds its way through latex gloves, dimethyl mercury penetrates the skin within seconds.
Her log book revealed that she only worked with the neurotoxin for one day. Four months after the single exposure from the spillage, she was admitted to the hospital suffering from a loss of speech, balance and gait. She had also lost weight and suffered from brief episodes of nausea and diarrhea. A blood analysis revealed 4 ppm of mercury, twenty times the toxic level. They tried chelation therapy, which attempts to chemically bond the metal for purposes of excretion. But regardless, because Hg metabolites had already bonded to proteins in her brain, it was too late. She eventually fell into a coma with spontaneous moments of agitation and crying.
On June 8, 1996, on the day my daughter was born, Karen Wetterhahn died at the age of 48.
Of the literally millions of compounds that have been made in the last hundred years, fortunately the supervillains are in the minority. But there's no magical way of clearly categorizing them as such when they are first synthesized. The same applies to those of the third category: the variety of uses emerge with time, and the choices only become possible after we learn from a trial and error method that results in victims.